All Solutions

Explore all the solutions you can create with Paperform: surveys, quizzes, tests, payment forms, scheduling forms, and a whole lot more.
See all solutions










Connect with over 2,000 popular apps and software to improve productivity and automate workflows
See all integrationsProducts
Solutions
All Solutions

Explore all the solutions you can create with Paperform: surveys, quizzes, tests, payment forms, scheduling forms, and a whole lot more.
See all solutionsIntegrations

Connect with over 2,000 popular apps and software to improve productivity and automate workflows
See all integrationsResources
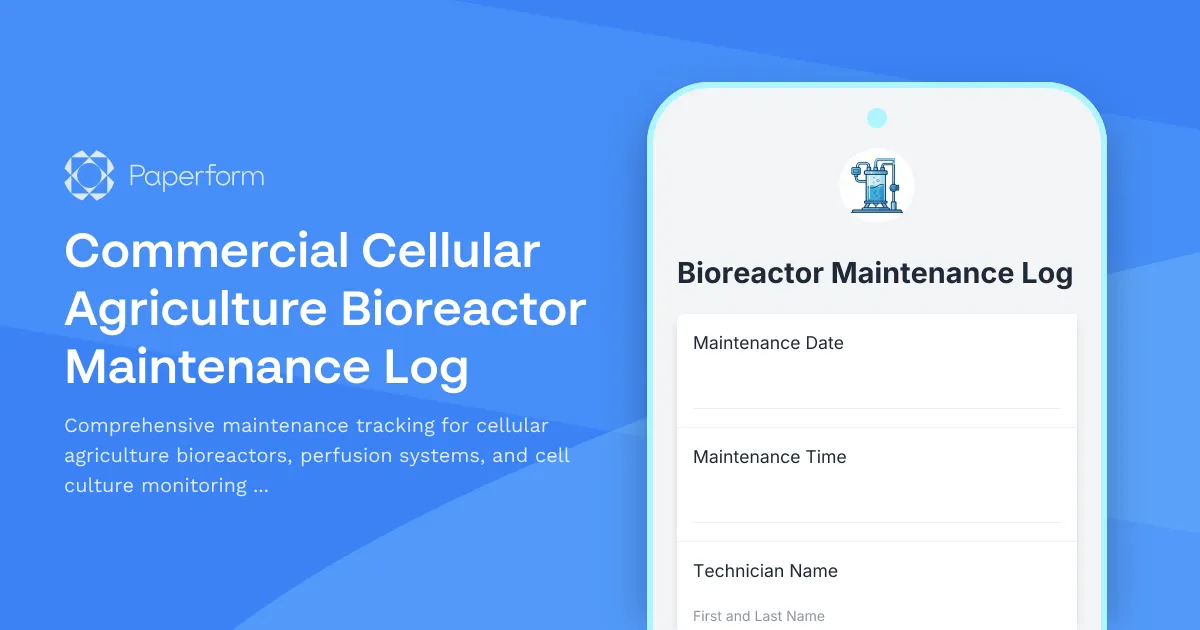
Commercial Cellular Agriculture Bioreactor Maintenance Log Template
Maintaining critical cell culture infrastructure requires meticulous documentation and regular calibration checks. This Commercial Cellular Agriculture Bioreactor Maintenance Log template provides a comprehensive solution for tracking all maintenance activities across your bioprocessing equipment—from perfusion system calibration to dissolved oxygen sensor verification.
Why proper bioreactor maintenance tracking matters
In cellular agriculture and cultivated meat production, bioreactor performance directly impacts cell yield, product quality, and regulatory compliance. Equipment failures or calibration drift can compromise entire production batches, making systematic maintenance documentation essential for:
- Regulatory compliance: FDA, USDA, and international food safety authorities require detailed equipment maintenance records
- Quality assurance: Traceable calibration data ensures consistent cell culture conditions
- Predictive maintenance: Historical logs help identify patterns and prevent costly downtime
- Audit readiness: Complete maintenance history supports GMP and HACCP certifications
Built for bioprocessing operations teams
This template is specifically designed for cellular agriculture facilities, biotech manufacturers, and R&D labs running commercial-scale cell culture operations. Whether you're producing cultivated meat, precision fermentation products, or other cell-based materials, this log captures the critical maintenance activities that keep your bioreactors running at optimal performance.
Key features include:
- Perfusion system calibration tracking: Document flow rates, pump performance, and media exchange parameters
- Dissolved oxygen sensor verification: Record sensor readings, calibration standards, and accuracy checks
- pH controller maintenance: Log calibration points, buffer solutions, and controller performance
- Cell culture monitoring equipment: Track CO₂ sensors, temperature probes, agitation systems, and more
- Preventive maintenance scheduling: Plan and record routine inspections before issues arise
- Corrective action documentation: Capture problems, solutions, and follow-up verification
Paperform: The ideal platform for biotech maintenance workflows
Paperform makes it easy for operations teams to maintain compliance-ready records without complex software. Unlike rigid CMMS platforms, Paperform's document-style editor lets you create a maintenance log that matches your exact SOPs and equipment specifications.
Streamlined data capture: Technicians can quickly log maintenance activities from any device—desktop computers in the lab, tablets mounted near equipment, or smartphones during facility rounds. Conditional logic shows only relevant fields based on equipment type and maintenance category, reducing form completion time and errors.
Automated workflows with Stepper: Connect your maintenance log to Stepper to automatically trigger follow-up actions when calibration values fall outside acceptable ranges, create work orders for corrective maintenance, notify supervisors of critical equipment issues, and update your CMMS or asset management system in real-time.
Calculations and validations: Built-in calculation fields can automatically flag sensor drift, calculate time since last calibration, and verify that readings fall within acceptable tolerances—helping technicians identify issues during the maintenance check rather than during post-hoc review.
Secure digital signatures with Papersign: For GMP operations requiring supervisor sign-off on maintenance activities, integrate Papersign to collect legally binding eSignatures on completed maintenance records. This creates a complete audit trail linking the maintenance log to formal approval, perfect for regulatory inspections.
Integration with your existing systems: Push maintenance data automatically to your laboratory information management system (LIMS), quality management system (QMS), or maintenance tracking software via native integrations with tools like Airtable, Google Sheets, or through Stepper's advanced workflow capabilities.
Perfect for multiple bioprocessing roles
This template serves various team members across your cellular agriculture operation:
Operations technicians complete routine maintenance checks efficiently with mobile-friendly forms that guide them through each verification step, ensuring nothing gets missed during busy production schedules.
Quality assurance managers access complete maintenance histories for batch release decisions, deviation investigations, and trend analysis—all stored centrally with timestamps and technician identification.
Compliance officers generate audit-ready documentation instantly, with searchable records showing equipment maintenance frequency, calibration traceability, and corrective action closure.
Maintenance coordinators schedule preventive maintenance based on equipment runtime hours, cycle counts, or calendar intervals, with automatic reminders ensuring critical calibrations never get overlooked.
Facility managers monitor equipment reliability across multiple bioreactor systems, identifying high-maintenance equipment that may need replacement or upgrade investment.
Trusted by biotech and cellular agriculture pioneers
Leading cellular agriculture companies, contract development and manufacturing organizations (CDMOs), and biotech research facilities trust Paperform to handle their critical documentation workflows. With SOC 2 Type II compliance, data residency controls, and enterprise-grade security, Paperform provides the reliability that bioprocessing operations demand.
Our platform scales from single-bioreactor pilot facilities to multi-suite commercial production plants, with Agency+ features for contract manufacturers managing documentation across multiple clients.
Get started in minutes
This template includes all the essential maintenance categories for cellular agriculture bioreactor systems, but Paperform's flexible editor makes it easy to customize fields for your specific equipment brands, SOPs, and regulatory requirements. Add your facility's logo, adjust acceptance criteria, incorporate equipment-specific calibration procedures, or remove sections that don't apply to your operation.
Whether you're maintaining stirred-tank bioreactors, perfusion systems, single-use bioreactor platforms, or custom cell culture vessels, this template provides the documentation framework you need to keep your cellular agriculture operation running smoothly and compliance-ready.
Start tracking your bioreactor maintenance with Paperform today—no complex implementation, no IT project required, just professional maintenance documentation that works.