All Solutions

Explore all the solutions you can create with Paperform: surveys, quizzes, tests, payment forms, scheduling forms, and a whole lot more.
See all solutions










Connect with over 2,000 popular apps and software to improve productivity and automate workflows
See all integrationsProducts
Solutions
All Solutions

Explore all the solutions you can create with Paperform: surveys, quizzes, tests, payment forms, scheduling forms, and a whole lot more.
See all solutionsIntegrations

Connect with over 2,000 popular apps and software to improve productivity and automate workflows
See all integrationsResources
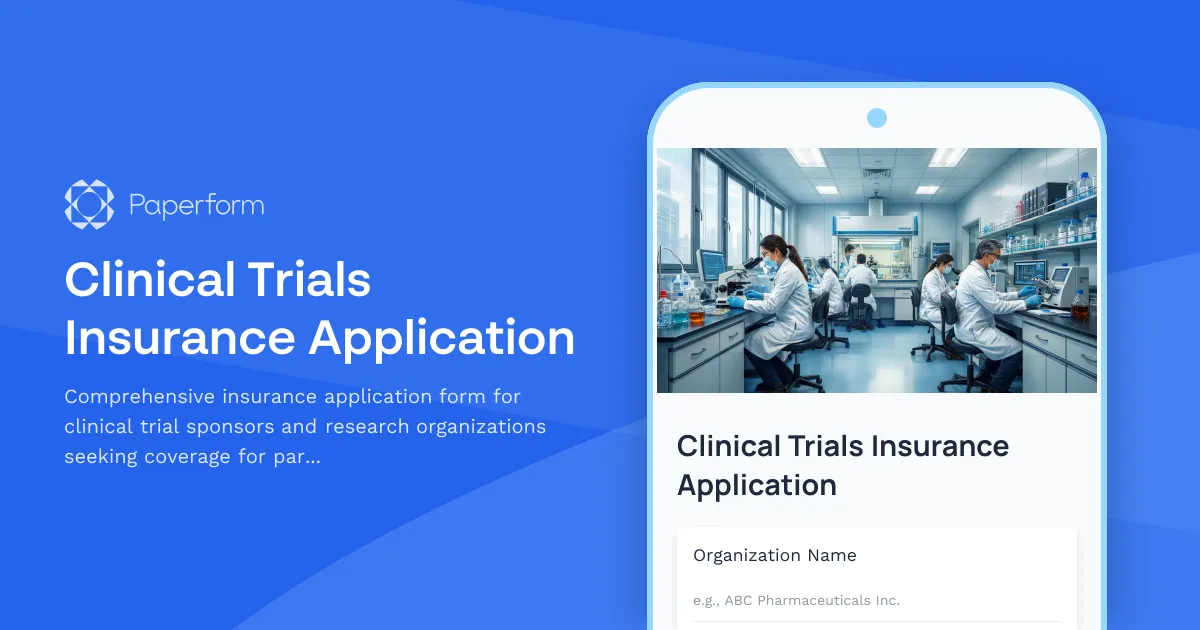
Clinical Trials Insurance Application Form Template
Running a clinical trial involves significant responsibility—especially when it comes to protecting participants, researchers, and your organization from potential liability. Whether you're a pharmaceutical company, research institution, or contract research organization (CRO), securing the right insurance coverage is a critical regulatory and ethical requirement.
This Clinical Trials Insurance Application template from Paperform streamlines the complex process of applying for clinical trial insurance. Instead of wrestling with lengthy PDF forms or disconnected email chains, you can collect all the essential information—study protocols, participant demographics, adverse event history, regulatory compliance documentation, and risk assessments—in one professional, easy-to-complete form.
Why clinical trial insurance matters
Clinical trials insurance typically covers three main areas: participant liability (compensation for trial-related injuries), investigator indemnity (protection for principal investigators), and sponsor liability (coverage for the organization running the trial). Securing adequate coverage requires detailed disclosure about your study design, phase, intervention type, participant population, and historical safety data.
Insurance underwriters need comprehensive information to assess risk accurately, and incomplete applications lead to delays, back-and-forth requests, and potential coverage gaps. This template helps research organizations submit complete, accurate applications the first time, accelerating the approval process and ensuring trials can commence on schedule.
Who this form is for
This template is designed for:
- Clinical research organizations (CROs) managing trials on behalf of sponsors
- Pharmaceutical and biotech companies conducting drug or device trials
- Academic medical centers and research institutions running investigator-initiated studies
- Insurance brokers specializing in clinical trials and life sciences coverage
- Regulatory affairs teams coordinating compliance and risk management documentation
Whether you're running a Phase I first-in-human study or a large Phase III multinational trial, this form adapts to capture the specific details insurers require.
What this template includes
The Clinical Trials Insurance Application collects:
- Organization and contact information for the sponsor, principal investigator, and insurance broker
- Study protocol details including trial phase, indication, intervention type, and study design
- Participant information including enrollment numbers, demographics, inclusion/exclusion criteria, and informed consent procedures
- Adverse event history from previous trials or preclinical studies
- Regulatory compliance status including IRB/ethics committee approval, regulatory authority submissions, and data safety monitoring plans
- Risk assessment covering known risks, mitigation strategies, and safety monitoring protocols
- Coverage requirements specifying the types and amounts of insurance needed
- Supporting documentation uploads for protocols, investigator brochures, informed consent forms, and safety reports
The form uses conditional logic to show or hide relevant fields based on trial phase, intervention type, and previous adverse event history—so applicants only see questions that apply to their specific study.
Streamline applications with Paperform's professional features
Paperform makes clinical trials insurance applications significantly easier to manage:
Branded, professional experience: Customize the form with your organization's colors, logo, and fonts so it feels like a natural extension of your research operations. Embed it directly on your intranet or insurance portal, or send it as a standalone link.
Conditional logic for complex workflows: Show different questions based on trial phase (Phase I vs Phase III requires different disclosures), intervention type (drug vs device vs biologic), or whether there's a history of serious adverse events. This keeps forms lean while ensuring completeness.
Secure file uploads: Applicants can attach study protocols, investigator brochures, informed consent templates, preclinical safety data, and regulatory correspondence directly within the form. Everything stays organized and linked to the application.
Calculations for participant numbers and coverage: Use built-in calculations to automatically compute total participant exposure (participants × trial duration), required coverage amounts based on phase and risk category, or premium estimates based on underwriting criteria.
Integration with your systems: Send completed applications directly to your CRM, project management tool, or document repository. Connect to HubSpot, Salesforce, Airtable, Google Sheets, or use webhooks to push data wherever it needs to go. For insurance brokers managing multiple clients, this eliminates manual data entry entirely.
Papersign for policy agreements: Once coverage is approved, use Papersign to turn the application into a binding policy document and collect eSignatures from all required parties—sponsors, investigators, and insurers—keeping everything linked for a complete audit trail.
Stepper for workflow automation: Use Stepper, Paperform's AI-native workflow builder, to automate what happens after submission. Route applications to underwriting teams, trigger compliance reviews, send automatic follow-ups for missing documentation, update internal tracking systems, and notify all stakeholders when policies are issued—all without manual handoffs.
Better applications, faster approvals, safer trials
For clinical research organizations and insurance brokers, this template transforms a typically cumbersome application process into a streamlined, professional experience. Research teams can complete applications in one sitting with all necessary documentation at hand, reducing back-and-forth and accelerating time to coverage.
For insurance underwriters, receiving structured, complete data means faster risk assessment and more accurate pricing. For trial sponsors, having insurance in place on schedule means trials can start on time, participants are properly protected, and regulatory requirements are met.
Paperform is trusted by over 500,000 teams worldwide and is SOC 2 Type II and GDPR compliant, giving research organizations and insurance providers the security and reliability they need for sensitive clinical trial information.
Get started with this Clinical Trials Insurance Application template today and bring structure, speed, and professionalism to your clinical trial risk management process.