All Solutions

Explore all the solutions you can create with Paperform: surveys, quizzes, tests, payment forms, scheduling forms, and a whole lot more.
See all solutions










Connect with over 2,000 popular apps and software to improve productivity and automate workflows
See all integrationsProducts
Solutions
All Solutions

Explore all the solutions you can create with Paperform: surveys, quizzes, tests, payment forms, scheduling forms, and a whole lot more.
See all solutionsIntegrations

Connect with over 2,000 popular apps and software to improve productivity and automate workflows
See all integrationsResources
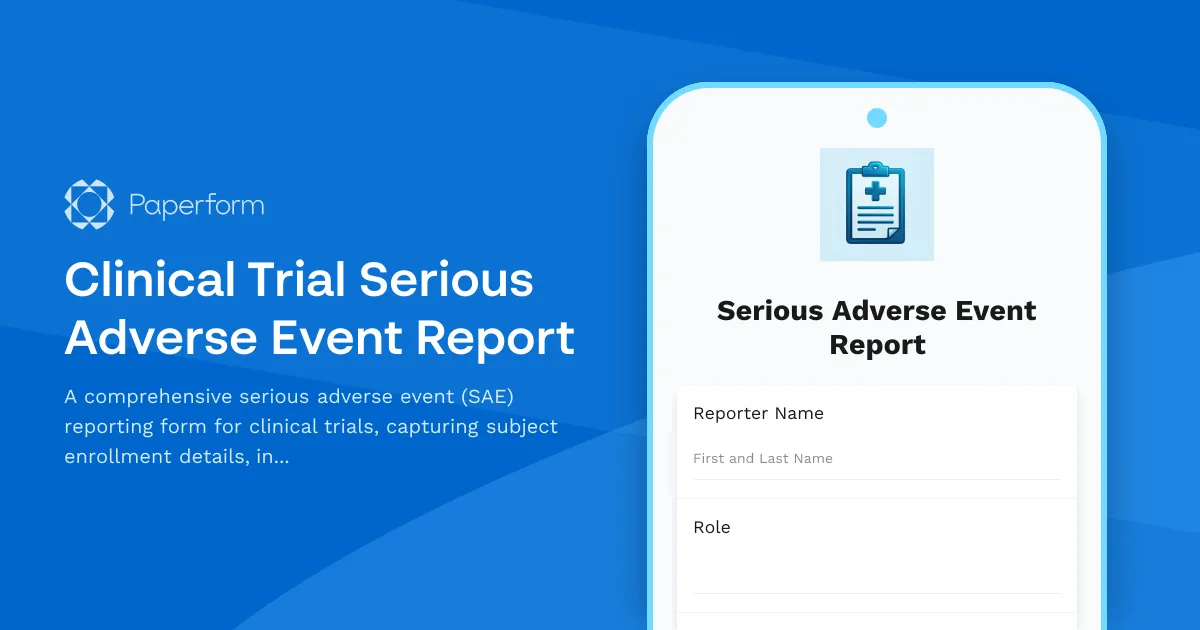
Clinical Trial Serious Adverse Event Report Template
Running clinical trials requires meticulous safety monitoring and rapid, accurate reporting of serious adverse events (SAEs). When an unexpected medical event occurs during a trial, investigators need a standardized way to capture comprehensive details, assess causality, determine reporting obligations, and ensure regulatory compliance—all while working under tight timelines.
This Clinical Trial Serious Adverse Event Report template from Paperform gives research coordinators, clinical trial managers, principal investigators, and pharmaceutical safety teams a professional, compliant form for documenting SAEs from initial detection through final resolution. Whether you're conducting Phase I–IV trials, managing multi-site studies, or coordinating CRO operations, this template helps you meet FDA, ICH-GCP, and IRB reporting requirements without the complexity of legacy clinical trial management systems.
Built for clinical research workflows
The template walks through every critical component of SAE reporting: subject enrollment and demographics, event description and severity grading, investigator causality assessment, expectedness determination, regulatory notification timelines, and outcome tracking. Conditional logic ensures the right follow-up questions appear based on event seriousness, relationship to study intervention, and regulatory thresholds—so your team captures exactly what's needed for FDA MedWatch, sponsor safety databases, and IRB continuing review.
By digitising SAE reporting in Paperform, you replace error-prone PDFs and email chains with a single source of truth that timestamps every submission, validates required fields, and routes urgent reports to the right stakeholders immediately. Forms can be embedded directly into your trial site portal or shared as secure links for investigator access.
Automate regulatory workflows with Stepper
After an SAE is submitted, time-sensitive actions need to happen fast. Connect this form to Stepper, Paperform's AI-native workflow builder, to automatically notify the sponsor safety team, generate draft MedWatch 3500A reports, update your clinical trial management system (CTMS), trigger IRB notification emails based on relatedness and expectedness, and escalate life-threatening events to the medical monitor—all without manual handoffs. Stepper keeps your safety reporting compliant, auditable, and on schedule.
Designed for healthcare and pharmaceutical teams
This template is ideal for clinical research organisations (CROs), pharmaceutical and biotech sponsors, academic medical centers, site management organisations (SMOs), contract research site networks, principal investigators, and regulatory affairs teams managing safety data across trials. It supports the rigorous documentation standards required by FDA 21 CFR Part 312, ICH E2A guidelines, and institutional review board (IRB) policies, helping you maintain Good Clinical Practice (GCP) compliance at every site.
With Paperform's SOC 2 Type II compliance, secure data handling, and powerful integrations, you can confidently digitise serious adverse event reporting and connect it to the broader clinical trial ecosystem—from EDC systems to pharmacovigilance databases—without sacrificing speed, accuracy, or regulatory readiness.